The following text is the press release article I wrote few years ago upon request of Media Relations of University of California, Irvine, shortly after I started working for them. Finally, it hadn’t been used because the article was based on my scientific results from Fritz-Haber Institute of Max Planck Society, then published in the Science magazine (Science 304 (2004), pages 1639-1644) under the title „Fluctuations and Bistabilities on Catalyst Nanoparticles„.
Since the press release was written with quite a general public in mind I tried to provide enough simple background so that most people could understand at least the main idea of our science findings.
Fluctuations and Bistabilities on Catalyst Nanoparticles

Although the most of people even do not realize it catalysts play an irreplaceable role in our lives. Catalysis is not an industry by itself but rather a key technology used by many industries. General importance of catalysis is, e.g. for petroleum working industry, automotive industry, biochemistry, pharmaceutic industry, fuel cells development etc. The number of catalytic materials applied in industry is very large and catalysts come in many different forms, e.g. powders, spheres, tablets, wires, and other solid forms as well as a coating. Among the most commonly used are heterogeneous catalysts in the form of inert porous solids or powders covered by highly dispersed chemically active metal (typically precious metals like platinum, palladium, and rhodium).
What exactly is catalysis about? Catalysis is a phenomenon by which a relatively small amount of a substance, called a catalyst, accelerates a chemical reaction without itself being consumed by the reactions they aid. Moreover, catalysts not only enhance the rates of reaction (=effectiveness), but they also direct reactants to specific products (=selectivity).
Catalysis generally means the reduction of the activation energy required for a reaction. Realizing this fact the importance of the catalysis appears to be obvious: The less energy required means that a reaction can take place at lower temperatures and pressures which results in savings in terms of energy, raw materials and plant and process costs as well as in higher yields through active control of the reactions. Moreover, the presence of a catalyst can make possible a reaction that would not run otherwise. That is why the catalysis is extremely attractive not only from economic but also from environmental considerations.
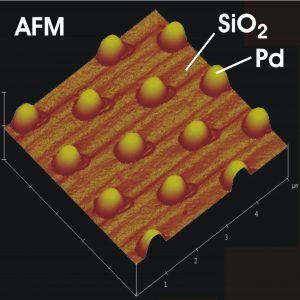
The pure metals are usually not capable of catalyzing the reaction with the desired selectivity and effectiveness. Therefore a certain „fine-tuning“ has to be done, where the active metal has to be adapted to the specific application. In this report we focus to so-called heterogeneous catalysis where the catalyst has a different phase (typically solid) than the reactants (usually gas or liquid). The well-known example of heterogeneous catalyst is the automotive exhaust converter where porous oxide structure is covered by finely dispersed particles of platinum or rhodium or their combination.
One of the most fascinating aspects of heterogeneous catalysis is that it is largely an empirical science. The application of catalysis has been a necessity for the chemical industry for at least 150 years, while the experimental techniques for investigation of catalysis at the atomic level did not become routine until less than 25 years ago. Large amounts of empiric knowledge exist that awaits systematic investigation. A huge barrier in better understanding comes from the vast complexity of real catalysts which is not directly accessible by existing experimental techniques at microscopic level. Thus the catalytic phenomena are usually studied on the model systems with strongly reduced complexity but still highly relevant to the realistic structures.
In our case, the model catalyst consisting of small metal islands deposited on the surface of a perfectly flat aluminum and silicon oxide has been used. We compared different samples with average lateral size of metal particles varying between the few to several hundreds of atomic diameters (2 to 500 nanometers).
The investigation of the mechanisms of the catalysis is highly motivated by the desire to make development of the novel catalysts and optimization of the existing ones more rational and efficient. Appreciable progress has been done in last two decades and there were theories and models developed in order to explain and predict the elementary processes during catalyzed reaction. However, there are several phenomena remaining which cannot be explained by the present theories.
One of the much discussed „mysteries“ in heterogeneous catalysis are so-called size effects, i.e. the role of the size of the active metal particles. Size effects are a common phenomenon and are typically taken advantage of in catalyst optimization. Just by changing the size of the metal particles their chemical properties may vary in very broad range despite the elemental composition of the catalyst is unaffected. It is because each particle is the ensemble of atoms which are interacting between each other. The physical and chemical behavior of a single atom is different from behavior of the cluster of many atoms especially because of the mutual bonds they form to their neighbors. The less atoms are in a single cluster the more similar to a single atom they behave and vice versa. By changing the number of atoms in a metal particle catalytic properties can be „tuned“.
Unfortunately, the exact origin of size effects remains highly ambiguous in most cases. In our paper we are revealing a part of the „mystery“ around heterogeneous catalysis which arises as a consequence of the limited dimension of the active particle. We focused on a general but widely ignored „nanoscale“ effect, which is the influence of fluctuations of molecules present in a chemical reaction on the surface of the catalyst. During the reaction all molecules (both reactants and products) sitting on the active surface of the catalyst (so called adsorbed molecules) continually change their actual positions due to the thermal vibrations generally present in any matter, in other words they fluctuate their local density. This movement is responsible for rapid rearrangement of the adsorbed molecules and their random motion across the surface (so called surface diffusion) which can be addressed to a kind of microscopic „communication“ between different areas of the catalyst.
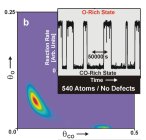 Although this movement is extremely fast it occurs on a very small scale in the order of size of an atom. Thus, if the metal particle is sufficiently big there may coexist different spots on it which do not „communicate“ to each other efficiently because the molecule originally located on one side of the particle is consumed by the reaction before it reaches the other side. It means that, in general, we can have different physical conditions on the same single particle (e.g. different absolute density of the adsorbed molecules, different abundance of reactants and products, etc.). Accordingly on the particular catalyst one can observe reaction running in more different regimes in the same time. This effect is called multistability (or bistability in the case of coexistence of just two reaction regimes).
Although this movement is extremely fast it occurs on a very small scale in the order of size of an atom. Thus, if the metal particle is sufficiently big there may coexist different spots on it which do not „communicate“ to each other efficiently because the molecule originally located on one side of the particle is consumed by the reaction before it reaches the other side. It means that, in general, we can have different physical conditions on the same single particle (e.g. different absolute density of the adsorbed molecules, different abundance of reactants and products, etc.). Accordingly on the particular catalyst one can observe reaction running in more different regimes in the same time. This effect is called multistability (or bistability in the case of coexistence of just two reaction regimes).
Besides the particle size its microscopic structure plays an important role. It is obvious that movement of the molecule on the surface is different depending if the surface is perfectly flat or it consists of many defects like steps, edges, bumps etc. Usually the smaller is the particle the higher density of such defects and other irregularities is observed.
According to what has been said about the relation between the magnitude of surface fluctuations and the occurrence of multistability on the catalyst it implicates that when the intensity of fluctuations is increased to a certain level the multistability will vanish because of increased intensity of „molecular communication“. It means that just by changing the size of the active metal particles we can obtain a catalyst that exhibits different behavior towards the particular chemical reaction. This is a fact commonly accepted, however, in former experimental studies performed by other research groups the possible role of the surface fluctuations has not been considered. Taking fluctuations into account can help us explain some of the discrepancies and „mysterious phenomena“, which people involved in catalytical chemistry have been experiencing every day.
The results we reported should likely hold not only for the specific case we studied, but also for other reactions exhibiting similar kinetic multistabilities. Besides that, our findings are consistent with previous theoretical predictions which have never been confirmed experimentally before, though. Regarding the crucial importance of the rational approach in modern catalysis mentioned above the implications of our findings for the industry and the environment are quite obvious.
